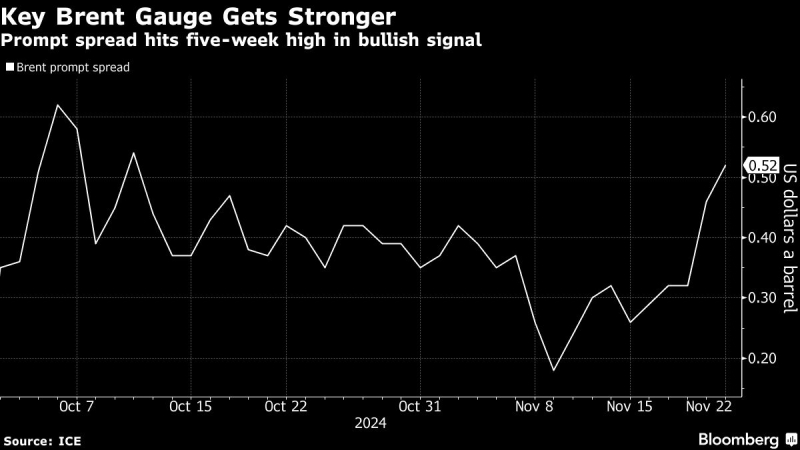
The U.S. Food and Drug Administration delivered a burst of hope to the roughly 6 million Americans currently living with Alzheimer's on Monday, announcing — for the first time in 20 years — the approval of a drug to fight the disease. The new therapy will be sold under the brand name Aduhelm and delivered by infusion every four weeks. How well the drug itself (technically known as aducanumab) works, however, remains somewhat of a mystery.
In a statement, the director of the FDA's Center for Drug Evaluation and Research, Dr. Patrizia Cavazzoni, expressed cautious optimism about aducanumab. "Currently available therapies only treat symptoms of the disease; this treatment option is the first therapy to target and affect the underlying disease process of Alzheimer's," said Cavazzoni. "As we have learned from the fight against cancer, the accelerated approval pathway can bring therapies to patients faster while spurring more research and innovation."
The drug has received accelerated approval from the FDA, an expedited green light given to drugs that "treat serious conditions" and "fill an unmet medical need." But with just two studies on its effectiveness, questions remain as to whether the drug will make an impact.
Two experts, in speaking with Yahoo Life, argue that the FDA was right to issue approval and say it's better to give individuals a chance to try it. "There is a great unmet medical need and so many people are desperate for a treatment for Alzheimer’s disease," says Dr. Robert J. Vassar, the Davee professor of Alzheimer's research at Northwestern University Feinberg School of Medicine. "Aducanumab may be the first disease-modifying therapy for AD, but it may not work for everyone."
For the first time in 20 years, the FDA has approved a new treatment for Alzheimer’s disease. Experts say the drug works by removing a toxic protein. (Photo: Getty Images)
Dr. Ronald Petersen, director of the Mayo Clinic Alzheimer's Disease Research Center and the Mayo Clinic Study of Aging, agrees. "This is the first disease-modifying therapy that's been approved for Alzheimer's by the FDA, and that's huge," says Petersen. "That's huge for our patients and for the entire field. It's not without its qualifications and responsibilities, but it is a huge day for the patients with Alzheimer's."
Until now, there has been no treatment to stop or slow the progression of the disease, making it difficult not only for those diagnosed with Alzheimer's, but the loved ones who care for them until the end. Now Vassar, who watched his mother battle the disease in her early 60s, says that could be changing. "[I] and a number of my colleagues are kind of on the fence about the approval," he says, mentioning the need for more data. "But my feeling is that the antibody approach to remove amyloid from the brain makes sense."
The "amyloid" he is referring to is a protein that clumps together in Alzheimer's patients' brains, blocking crucial pathways and eventually leading to cell death. The new drug — which contains an antibody that scientists isolated from elderly individuals that did not have Alzheimer’s disease — targets just that. "It makes sense that amyloid is a critical component of the disease process, and if you could prevent it from occurring or take it away at an early stage then I, and a large number of my colleagues, believe that should be beneficial for Alzheimer's disease," says Vassar.
The FDA says it will require Biogen, the creator of Aduhelm, to conduct a randomized controlled trial to see if the drug is effective. If it proves otherwise, the organization may consider revoking approval. Both experts seem to be hopeful that is not on the horizon. In fact, Vassar theorizes that it may be preventive. "Would it work if you went even earlier in disease? I think it would work even better," Nassar says. "So in individuals that were asymptomatic with normal memory … but had amyloid in the brain, they would be on the pathway to Alzheimer's. The drug would probably be even more effective at preventing the disease from occurring."
Petersen says the news is extremely positive but wants to temper enthusiasm. "Our responsibility as clinicians is to modify expectations. Again, it's not a cure, it's not going to reverse people's symptoms," he says. "It may only apply to a subset of people with Alzheimer's disease, that is people with more severe disease may not respond to it." Vassar echoes his thoughts. "It's not going to be the silver bullet for everyone, it's not going to be effective on everyone," he says. "It won't be a complete 100 percent success, but it may be beneficial for people in the earlier stages."
Want lifestyle and wellness news delivered to your inbox? Sign up here for Yahoo Life's newsletter.